Water: Its Structure and Properties
Water occurs in huge abundance on the Earth, where most of it lies in the oceans that cover 71% of its surface to an average depth of ~ 6 km. All three forms of water - ice, liquid, and vapor - are found.
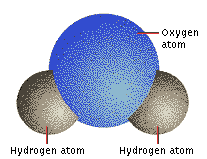
The oddest property of water is that it is a liquid at room temperature. This is surprising since the H2O molecule is so small that it would be expected to be a gas, like ammonia NH3, methane CH4, and its even closer relative hydrogen sulfide H2S. Waterís liquidity stems from the joint presence of the tiny hydrogen atoms, the lone pairs of electrons on the oxygen atom, and the power of the oxygen atom to attract electrons strongly. The oxygen attracts electrons so strongly that it draws the shared electron pair toward itself, leaving the positive charge of the hydrogen nucleus almost fully exposed. That exposed positive charge is strongly attracted to other electrons nearby, particularly those of oxygen atoms in other water molecules. These features cause water molecules to be strapped together by networks of hydrogen bonds. As a result, water occurs as a liquid under normal conditions on Earth. The molecules cluster together as a mobile liquid, rather than move independently as a gas.
Waterís oddness does not end with its liquidity. Most solids are more dense than the liquids from which they freeze, but ice at 0oC is less dense than water at OoC. As a result, ice floats on water, giving us icebergs and a solid skin on frozen ponds. As liquid water is warmed from 0oC its density initially increases, whereas most other substances become less dense as they warm. This anomalous increase continues until density reaches a maximum value at 3.98oC; beyond this the density decreases with temperature as in most other substances. (See Appendix 14 of Fetter.) This quirk of density is again due to hydrogen bonds, for when water freezes, its molecules are held apart, as well as held together, by the hydrogen bonds between them: Each molecule grips its neighbors firmly, but at armís length. The (hexagonal) structure of the solid is therefore more open than that of the liquid, in which many of the hydrogen bonds have collapses, so that it is less dense.
Water is an excellent solvent. It mixes readily with sugar, salt, and other minerals. This occurs because the water molecules simulate the surroundings of the ions in the crystals. Water is also a perfect medium for such processes as the transport of nutrients into cells. It can transport organic molecules like glucose, and the ions of such elements as sodium, potassium, and calcium that are so essential to an organismís functioning. Moreover, water, when it is a liquid, can do all this at body temperature.
In hydrogeologic settings, water is transported through the pore space of the aquifer. In addition to the density, two physical properties (compressibility and viscosity) of water play important role in controlling the hydraulic transport process. At room temperature (15.5oC), the compressibility of water is 4.4 x 10-10 m2 N-1 (or 4.4 x 10-10 Pa-1), and its viscosity is 1.124 x 10-3 N s m-2 s (or 1.124 x 10-3 Pa s = 0.01124 Poise). (See Appendix 14 of Fetter.) In spite of the strength of the hydrogen bonds, waterís viscosity is relatively low because of the rapidity with which the hydrogen bonds break and reform (about once every 10-12 s). Another important physical property is the surface tension, which controls the capillary action in the unsaturated zone. As might be expected from the strong intermolecular forces, water has a surface tension higher than most other liquids; its value at 0oC is 0.0756 Nm-1 (= 75.6 dyne cm-1). Compressibility increases and viscosity decreases with increasing temperature. Surface tension decreases rapidly as temperature increases. Dissolved substances can increase or decrease surface tension, and certain organic compounds have a major effect on its value.
References: Atkins, P. W., Molecules, W. H. Freeman and Company, 1987.
Dingman, S. L., Physical Hydrology, Macmillan, 1994